& Senior Co-Authorship
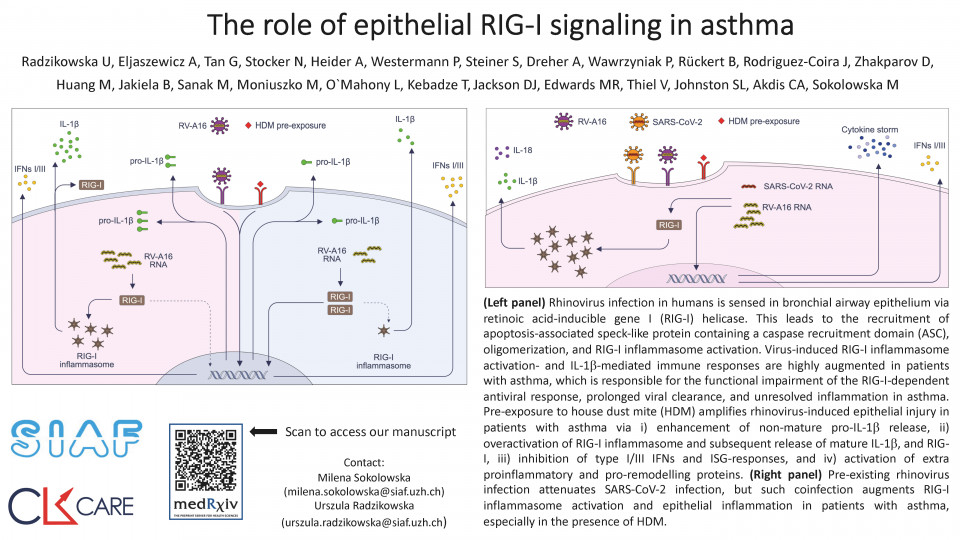
Rhinoviruses (RV) and inhaled allergens, such as house dust mite (HDM) are the major agents responsible for life-threatening asthma exacerbations. The mechanisms of this virus-allergen interactions in airway epithelium in asthma are largely unknown. To address this, we compared molecular mechanisms of HDM and RV interactions in experimental RV infection in patients with asthma and healthy individuals. RV infection was sensed via retinoic acid-inducible gene I (RIG-I) helicase, but not via NLR family pyrin domain containing 3 (NLRP3), which led to subsequent apoptosis-associated speck like protein containing a caspase recruitment domain (ASC) recruitment, oligomerization and RIG-I inflammasome activation.
This phenomenon was augmented in bronchial epithelium in patients with asthma, especially upon pre-exposure to HDM, which itself induced pro-IL-1b release and early inhibition of RIG-I/TANK binding kinase 1/IkB kinase e /type I/III interferons (RIG-I/TBK1/IKKe/IFN-I/III) responses. Excessive activation of RIG-I inflammasomes was partially responsible for alteration and persistence of type I/III IFN responses, prolonged viral clearance and unresolved inflammation in asthma. Finally, we investigated the role of severe acute respiratory syndrome coronavirus (SARS-CoV-2) in exacerbations of asthma or the influence of preexisting viral or allergic airway inflammation on the development of coronavirus disease 2019 (COVID-19).
We found, that RV/HDM-induced sustained IFN I/III responses initially restricted SARS-CoV-2 replication in epithelium of patients with asthma, but even this limited infection with SARS-CoV-2 augmented RIG-I inflammasome activation. In summary, timely inhibition of the epithelial IL-1b signaling may lead to more efficient viral clearance and lower the burden of RV and SARS-CoV-2 infection.