Der Vortrag wird auf Deutsch gehalten!
LEGO®-inspired multicomponent 3D-printed bone substitute for personalised facial bone repair
Introduction:
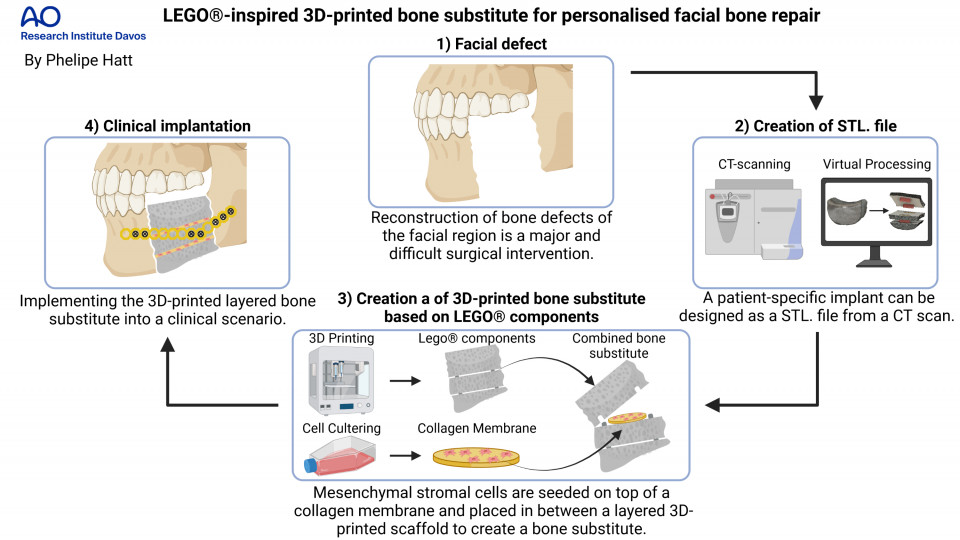
Large bone defects such as trauma, tumour resection, infections or congenital deformities do not have the capacity to heal inherently and therefore cause serious healthcare implications. Reconstruction of large bone defects in the facial area represents a challenging medical condition and requires the implantation of an autologous bone graft. This difficult surgical intervention includes harvesting a piece of bone of the patient's own body to be implemented into the defect site. Autologous bone grafting is the standard of care (SOC) to restore a functional and aesthetic outcome but is unfortunately associated with major drawbacks including limited availability, donor site pain and excessive graft resorption. An alternative SOC is urgently needed to overcome these issues. A tissue engineered approach combines the use of regenerative cells, a supporting scaffold material for mechanical stability and pro-osteogenic factors to create a bone substitute as an alternative. An attractive approach to create personalised scaffolds is the use of 3D printing. The main limitation of 3D-printed bone substitutes is the formation of a necrotic core upon implantation within the body, due to limited diffusion of oxygen and nutrients into the scaffold and poor biological waste removal. Also, delivery of regenerative cells within 3D-printed bone substitutes remains a challenge, due to the poor cell-adhesion properties of the scaffold. This work aims to create a 3D-printed personalised bone substitute based on a novel combination of materials, to form a scaffold with enhanced mechanical and cell-adhesion properties and with a configurable layered composition based on the LEGO® principle. The layered multicomponent approach allows for improved distribution of cells in the centre of the bone substitute by implementing collagen membranes that not only function as a collection chamber but also promotes cell viability and osteogenic cell response. Collagen is a very important protein for the early regeneration of bone that is produced by the bone cells and is therefore often used for bone tissue engineering.
Methods:
The newly developed printable ink is created by mixing a solvent, ethylene carbonate (EC), with poly(lactic-co-glycolic acid) (PLGA, 40% (w/V)), β-tricalcium phosphate (β-TCP, 20% (w/V)), and thermoplastic polyurethane (TPU, 10% (w/V)). EC is a non-toxic solvent that is water soluble which makes it easy to remove after printing. PLGA is a thermoplastic polymer that is biocompatible, bioresorbable and is added to improve printability of the ink and mechanical strength of the 3D-printed scaffold. β-TCP is composed of calcium phosphate, a brittle material with weak mechanical properties, but it provides the necessary osteoconductivity that promotes the formation of bone. TPU is a polymeric elastomer that is biocompatible and improves elastic mechanical properties. The LEGO®-like components are virtually designed using a CAD software (Autodesk Fusion 360®) and printed into personalised layered scaffolds via solvent-based printing using a RegenHu 3D Discovery® Bioprinter. Human bone marrow derived mesenchymal stromal cells (hBM-MSCs, obtained will full ethical approval) can differentiate into different cell types including bone cells and help with the regeneration of the lost bone tissue. Therefore, they are used in this study to test for the collagen membrane's osteogenic potential by undergoing an osteogenic differentiation experiment for 21 days on two commercially available collagen membranes: 1. Lyostypt (B. Braun) and 2. Collagen Cell Carrier (CCC) (Viscofan Bio Engineering). As last step, the viability of hBM-MSCs on the collagen membranes placed in between 3D-printed scaffold layers is examined after 8 days of cell culture.
Results:
Water-mediated solvent removal leads to surface microporosity and roughness, both confirmed by scanning electron microscope, and both favourable properties for improved cell-adhesion and osteogenic cell response. Mechanical compression and drilling tests of the 3D-printed scaffold show improved stiffness and destructibility compared to a commercially available ceramic. Under osteogenic culture conditions, hBM-MSCs placed on collagen membranes show satisfactory osteogenic outcome confirmed by upregulation of important bone markers such as alkaline phosphatase through protein expression and bone sialoprotein through gene expression. Cell viability assays show good hBM-MSCs cell survival on the Lyostypt cultured in between 3D-printed scaffold layers, while a higher number of dead cells were detected on the CCC.
Conclusion:
Large scale personalised bone substitutes can be successfully printed, assembled, and combined with cell-carrying collagen membranes within the LEGO®-inspired interlocking system. We propose a novel tissue engineered approach for large bone reconstruction as an alternative SOC to autologous bone grafting. Ongoing tests aim to demonstrate osteogenic capabilities of the 3D-printed scaffold itself.