Introduction
Intervertebral disc degeneration (IDD) and osteoarthritis (OA) are major causes of chronic pain and disability affecting the spine and joints. To date, long-lasting and effective treatments for them are lacking. Preclinical studies, investigating new treatments for IDD and OA, include cell and gene therapy, tissue engineering, and use of growth factors and small molecules. Elucidation of molecular mechanisms involved in these processes is crucial for the development of new treatments. Biological sex is a significant risk factor for both IDD and OA, which disproportionately and significantly more often affect women rather than men—probably because of the diversity related to hormonal and genetic differences. However, sex of cell donors or animals is often under-reported in preclinical studies, affecting the reproducibility and translational potential of research findings.
Methods
This study examined the reporting of biological sex in preclinical research articles related to IDD and OA published in high-impact journals in 2022. Articles included were sourced from very high-ranked journals (Q1 and Q2) in orthopaedics, rheumatology, and cell and tissue engineering categories. The inclusion criteria for articles comprised those that reported original preclinical studies involving the use of cells (in vitro), alive animals (in vivo) or tissue explants/organs from an organism in an external environment (ex vivo). We extracted data on whether the sex of the samples was reported, included in the analysis, and if the journals had requirements for sex reporting.
Results
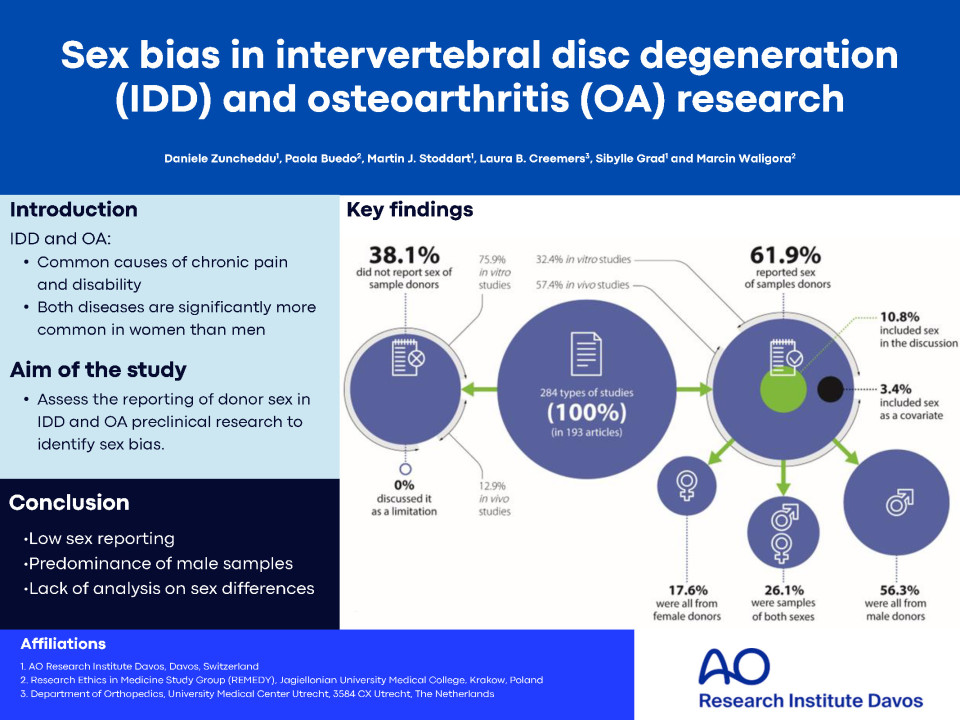
Of the 193 articles analysed, 73.6% were on OA and 26.4% on IDD. Of the study types, 61.9% reported the sex of the samples, but only 3.4% included sex as a variable in the analysis. Sex was reported more in in vivo studies than in in vitro or ex vivo studies: 57.4% versus 32.4% and 10.2%, respectively. Most journals (60.9%) required sex reporting through guidelines such as ARRIVE (Animal Research: Reporting of In Vivo Experiments), but only a few mentioned the SAGER (The Sex and Gender Equity in Research) guidelines specifically for sex and gender reporting. Additionally, we found misuse of the terms "sex" and "gender" in 7 articles, indicating a need for better understanding and training on these concepts.
Discussion
We identified four main concerns: low reporting of sample sex, predominance of male samples, lack of analysis considering sex differences, and reliance on external guidelines by journals. These issues can obscure important sex differences in disease mechanisms and hinder the development of effective treatments. Accordingly, setting tight guidelines on the reporting of sex by journals with advanced editorial standards should be done. Policy that promotes sex reporting in preclinical research has to be encouraged and underpinned by all funding agencies that are publicly funded.
Conclusion
The results show that a substantial number of pre-clinical research reported on IDD and OA under-reported biological sex. This is a contributor to its unsatisfactory translation to clinic trials and replication issues. Lessening sex bias within pre-clinical research is not only crucial for the sake of patients but also leads to an improvement in the quality and reproducibility of the research findings.
Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 955335.