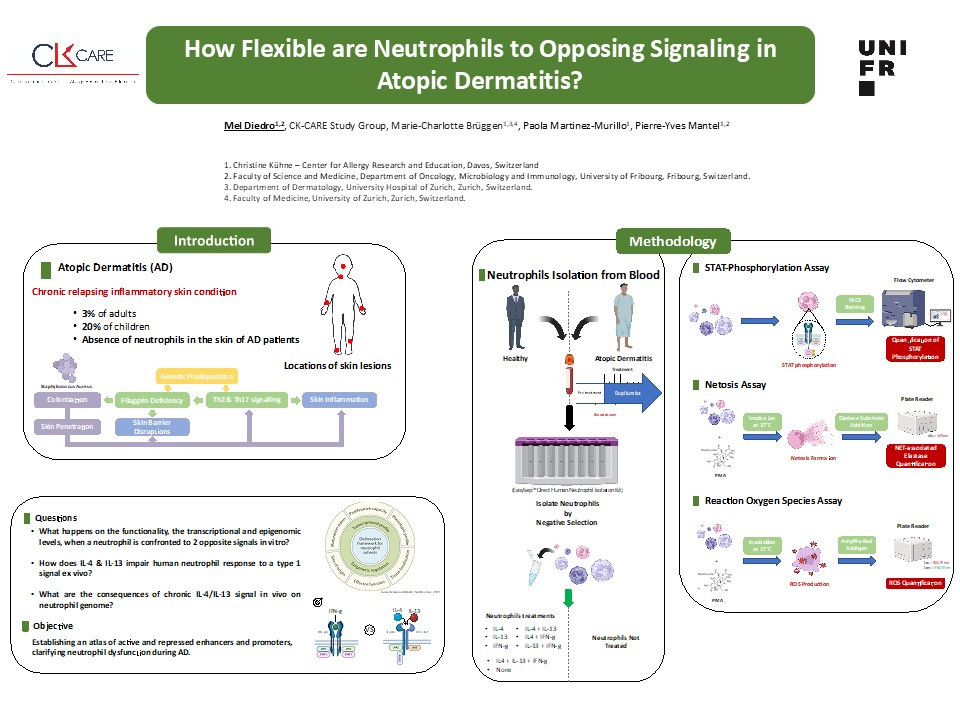
Atopic dermatitis (AD) is a chronic inflammatory skin disease with a heterogeneous clinical phenotype, affecting up to 20% of children and 3% of adults worldwide. Characterized by barrier dysfunction, persistent Th2 inflammation and skin dysbiosis due to Staphylococcus aureus overgrowth. Despite the overgrowth of pathogenic bacteria, skin lesions exhibit a conspicuous absence of neutrophils, potentially attributed to IL-4/13-induced suppression of neutrophil functions. Dupilumab is a game changer therapy this monoclonal antibody inhibits IL-4 and IL- 13 signaling by binding to the alpha subunit of the IL-4 receptor. However, concerns persist regarding its long-term effects and symptom recurrence upon cessation.
This study addresses a knowledge gap in neutrophil adaptation to an allergic environment, we investigate neutrophil adaptation to anti-bacterial response in a type 2 immune response dominated context such as AD by doing transcriptional and epigenomic profiling along with comprehensive analysis of neutrophil functionality (netosis, phagocytosis, ROS-production, bactericidal activity, chemotaxis). Preliminary data showed that upon in-vitro stimulation with IL-4, IFN-gamma or their combination, IL-4 induces STAT-6 phosphorylation and defective neutrophil response, while IFN-gamma induces STAT-1 phosphorylation and partially restores IL-4-induced dysfunction.
Building upon in-vitro stimulation insights, then we will aim for a comprehensive analysis of neutrophil functionality, open chromatin profiles, histone modifications, DNA methylation, and gene expression in healthy individuals and AD patients treated with Dupilumab. This analysis will create an atlas of active and repressed enhancers and promoters, clarifying their dysfunction during AD and advancing clinical strategies.