Introduction
In cartilage tissue engineering, a major challenge is that chondrocytes (cartilage cells) tend to lose their specific functions when grown on flat surfaces, making it difficult for them to form proper cartilage tissue. To address this, we developed a new type of hydrogel made from a special derivative of hyaluronic acid, which is a natural substance found in the body, and particles from decellularized extracellular matrix (dECM), which are derived from cartilage but have had all cells removed. This hydrogel aims to help maintain the chondrocyte's natural characteristics over time.
Methods
We prepared dECM particles by milling bovine cartilage (cow cartilage), treating it to remove DNA, and then grinding it into fine particles. These particles were mixed with chondrocytes and a special hyaluronic acid-based hydrogel, which was then solidified using enzymes and light, similar to how certain types of glue harden under specific conditions (Fig1).
For the in vitro experiments, different concentrations (6, 12 and 20%) of dECM particles were tested to reveal the optimal composition to maintain chondrocytes phenotype. For the ex vivo experiments, we created a cartilage defect in bovine osteochondral tissue samples and implanted the hydrogel-cell mixture inside these defects (Fig2).
The samples were cultured for 21 days, after which we analyzed the cell behavior, tissue structure, and hydrogel stability.
Results
The dECM particles retained a high percentage of important cartilage components like proteoglycans (64.3%) and collagen (97.3%), with most DNA removed (98.8%). Proteoglycans and collagen are crucial for maintaining the structure and function of cartilage. In the in vitro study, dECM particles upregulated the expression levels of important cartilage markers (ACAN, COL2, and SOX9) up to day 14, which are indicators of healthy cartilage formation. The group of 20% dECM particles showed the highest level of cartilage markers expression, which was selected for the following ex vivo study. After 21 days, the newly formed cartilage tissue within THA-20% dECM group explants (pieces of tissue) had higher glycosaminoglycans (GAG) and collagen content compared with the THA group, which are essential for cartilage elasticity and strength. At the same time point, the expression levels of ACAN, COL2, and SOX9 were higher in the 20% dECM explant hydrogels. THA-dECM explants group showed higher proteoglycan staining intensity compared with the THA groups.
Discussion & Conclusions
Adding dECM particles to the hydrogel made it more similar to natural cartilage, which helped chondrocytes maintain their functions better. This is important because chondrocytes need the right environment to stay healthy and produce good-quality cartilage. Culturing the hydrogel within osteochondral explants (bone and cartilage samples) improved its stability and reduced the loss of essential cartilage components like GAG and collagen, making the hydrogel more durable. This approach also positively regulated the chondrocyte characteristics, showing promise for future cartilage repair techniques. By closely mimicking the natural environment of cartilage, this method could lead to better outcomes in cartilage tissue engineering and repair.
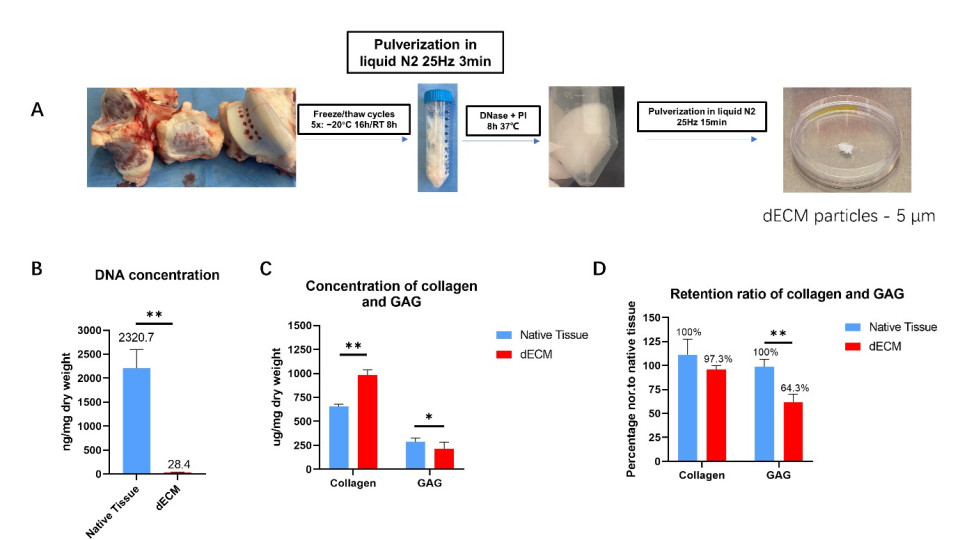
Fig. 1. (A) Macroscopic views of the production of dECM particles. (B) The DNA concentration, (C) Collagen and GAG concentration, and (D) retention ratio of collagen and GAG - (concentration x dECM yield dry weight) / (concentration x native tissue dry weight). Mean ± SD, n = 9, * p < 0.05, ** p < 0.01.
Fig. 1. (A) Macroscopic views of the production of dECM particles. (B) The DNA concentration, (C) Collagen and GAG concentration, and (D) retention ratio of collagen and GAG - (concentration x dECM yield dry weight) / (concentration x native tissue dry weight). Mean ± SD, n = 9, * p < 0.05, ** p < 0.01.
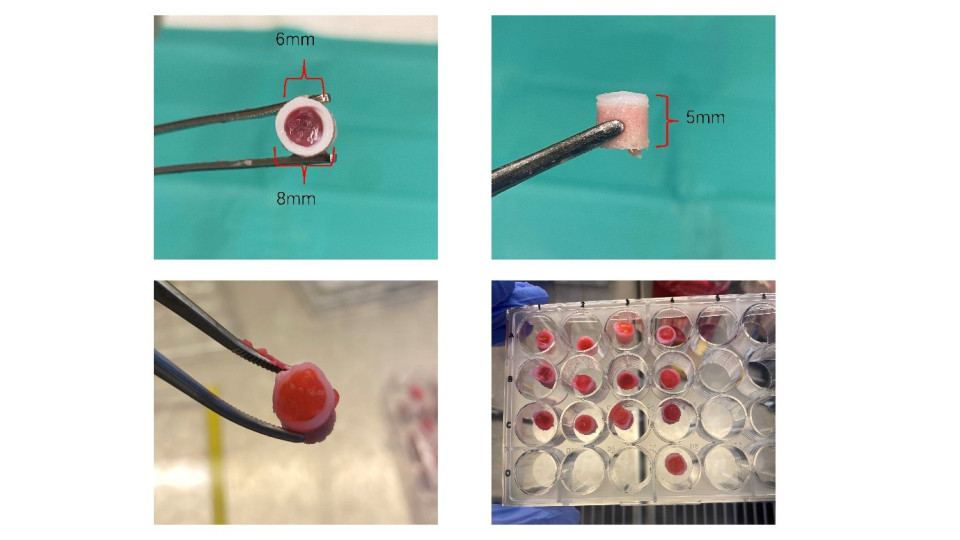
Fig. 2. a cartilage defect was created on osteochondral explants (8 mm diameter, 5 mm height) using 6 mm biopsy punch.
Fig. 2. a cartilage defect was created on osteochondral explants (8 mm diameter, 5 mm height) using 6 mm biopsy punch.