Eosinophilic esophagitis (EoE) is a chronic inflammatory disease characterized by esophageal inflammation, resulting in difficulty swallowing and esophageal dysfunction. It affects all age groups and the overall incidence rate has increased during the past decades overworld, and is currently estimated at 32.2 per 100,000 in Europe. Current available treatments include drugs such as topical corticosteroids, proton pump inhibitors, elimination diets, elemental diet and dilation therapy. Food and aeroallergens are the primary triggers of EoE which stimulate eosinophils, T cells, mast cell, dendritic cells and other inflammatory cells to express type 2-biased mediators and cytokines, impaired esophageal barrier and activated further inflammation. So far, research focused on the role of B cells and antibodies in EoE remains limited. However, it has been demonstrated that food specific antibodies are elevated both in serum and in esophageal biopsies of EoE patients. Indicating that, B cells, IgE and IgG4 may contribute to the pathophysiology of EoE.
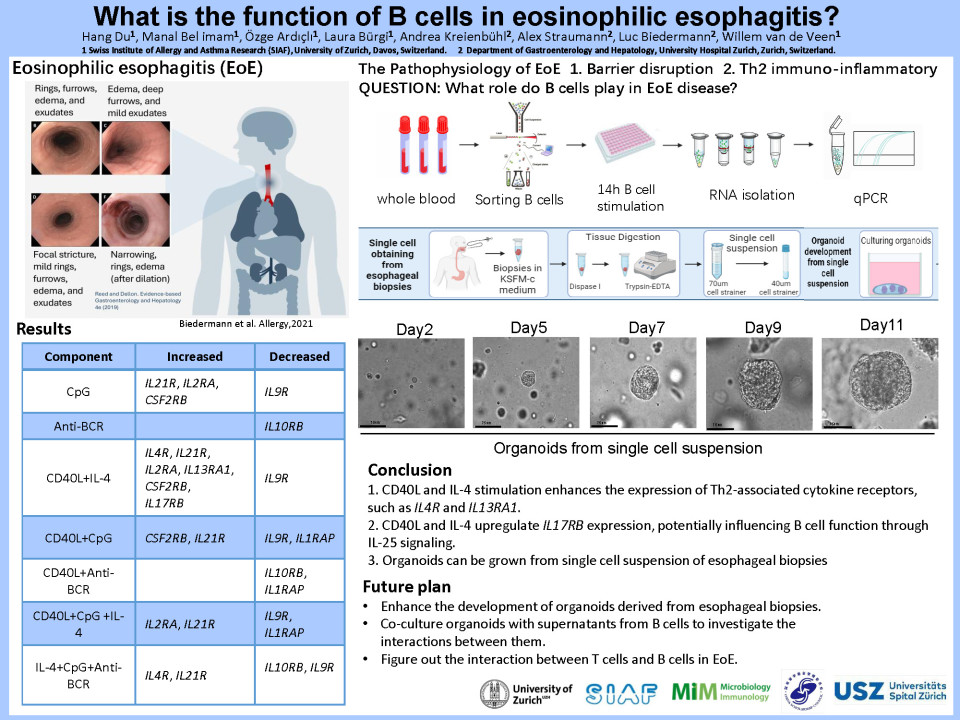
This project is focused on the role of B cells in eosinophilic esophagitis, the main purpose and methods of this research can be divided into the following parts: (1) Interrogation of interactions between B cells, T follicular helper cells and eosinophils in EoE in vivo and in vitro. To assess this, purified B cells subsets from PBMCs of healthy donors will be exposed to TSLP and IL-33 in combination with known B cell activation factors such as CD40L, CpG, BCR crosslinkers. We will use bulk RNAseq, flow cytometry, and targeted proteomics approached to assess the response of B cells to these stimuli. We will co-culture epithelial cell organoids with B cells and expose them to supernatants mentioned-above. To determine whether B cells in a type II environment have an effect on the epithelium, we will expose esophageal epithelial cells to supernatants of B cells stimulated with type-2 immune-cytokines.
We will analyze the effect on the epithelial barrier by transepithelial resistance (TEER) measurements using a microfluidics-based organ-on-a-chip system. The cellular response will be assessed through bulk RNAseq of esophageal epithelial organoids. (2) Exploring the interaction between TH2 and B cells in type II immune responses in EoE. We will assess the capacity of B cells to undergo classic switch recombination (CSR) to IgE and other immunoglobulin isotypes, plasma cell differentiation, and development of different effector B cell types. To achieve this, we will use B cells isolated from healthy donors as well as active and inactive EoE patients, stimulate with CD40L + IL-4 with or without IL-21 and with or without IL-9 and IL-10, Flow cytometry, ELISA RT-qPCR-based quantification will be used to analysis the changes and development of B cells. (3) Elucidate the pathophysiological mechanisms underlying Food-induced Immediate Response of the Esophagus (FIRE) which is a newly identified EoE phenotype.
A pilot project will provide FFPE esophageal biopsies from 53 EoE patients with and without FIRE, we will use more in-depth tissue analysis like visium spatial and Hyperion mass spec imaging to provide a more in-depth characterization of the immune cell composition and their spatial distribution in tissue.