Introduction
Osteoarthritis (OA) is a chronic degenerative joint disease which is currently one of the leading causes of disability in the elderly. Currently there is no effective cue for OA and the investigations for new therapeutics are limited due to the lack of accurate in vitro models.
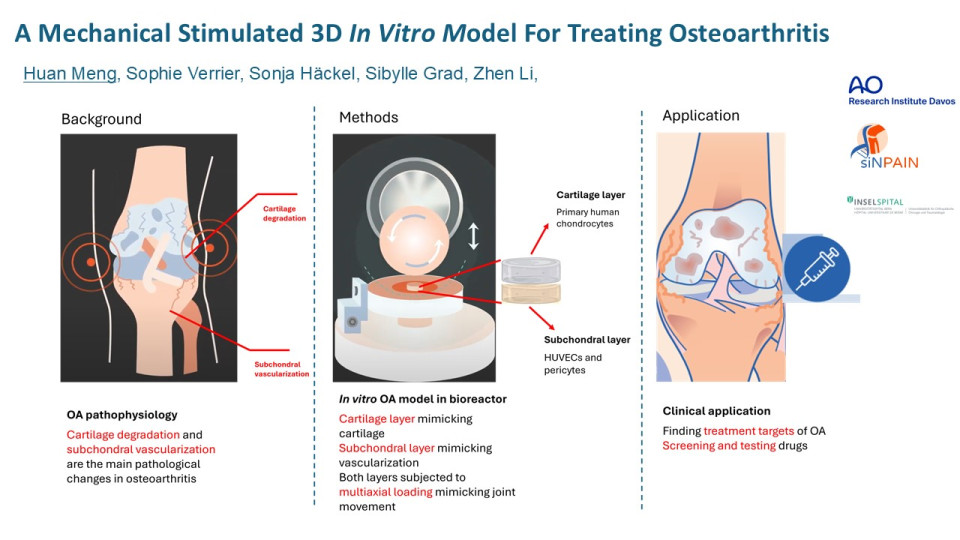
Recent studies showed that the pathological vascularisation in subchondral bone, as known as subchondral angiogenesis, plays a vital role in the degradation of cartilage tissue, which is the main pathological change in osteoarthritis. We hereby aim at developing a bilayer 3D OA model, containing a cartilage layer and a subchondral layer, as a platform for better understanding the OA mechanisms and accurately testing new targeted therapeutics.
Methods
For the development of the cartilage layer, chondrons were reproduced by stimulating pericellular matrix production of primary human OA chondrocytes in alginate gel. Then chondrons were harvested by dissolving the alginate gel and were characterised by immunofluorescent staining of collagen VI. The comparison of the isolated chondrocytes and the reproduced chondrons in the catabolic and chondrogenic activity as well as the inflammatory response to IL1β were tested at corresponding gene-expression level by qPCR technique.
For the development of the subchondral layer, primary human umbilical vein endothelial cells (HUVECs) were cultured in fibrin-polyurethane (PU) scaffolds +/- pericytes in Endothelial Growth Media 2 (EGM2). Then the vascularisation was analysed by the quantification of micro-tubular structures in histology images.
Finally, the coculture conditions were tested by combining cartilage layer and subchondral bone layer and cocultured in EGM2 media, chondropermissive (CP) media or 50%/50% EGM2/CP media. Cell morphology and viability were analysed by Calcein AM/ ethidium homodimer (EthD-1) assay.
Results
The reproduction of chondrons were confirmed by over 99% of chondrocytes with positive collagen VI staining. Compared to isolated chondrocytes, the reproduced chondrons in PU scaffolds showed significant elevated COL2 and ACAN expression in the absence of IL1β; while with IL1β treatment, the reproduced chondrons had upregulated COX2 expression.
Compared with HUVECs-scaffolds, the number and area of tubular structures showed a significantly increase in pericyte-HUVEC coculture scaffolds.
The chondrocytes and HUVECs/pericytes in coculture in CP/EGM2 mixed media did not show major viability loss.
Conclusion and discussion
In the cartilage layer, the reproduced chondrons in 3D scaffolds had elevated matrix production and upregulated inflammatory response. In the subchondral layer, coculture of pericytes significantly promoted angiogenesis of HUVECs. Coculture of both layers in mixed media did not show any compromised cell viability.
Therefore, our bilayer cartilage/subchondral coculture model offers a novel “joint-in-lab” platform for the deeper understanding of subchondral bone angiogenesis and cartilage degradation related OA mechanisms and for the development of targeted therapeutics. Future enhancements of our model will involve further optimisation of coculture conditions and the application of mechanical loading mimicking multiaxial joint movements, using our unique cartilage bioreactor.