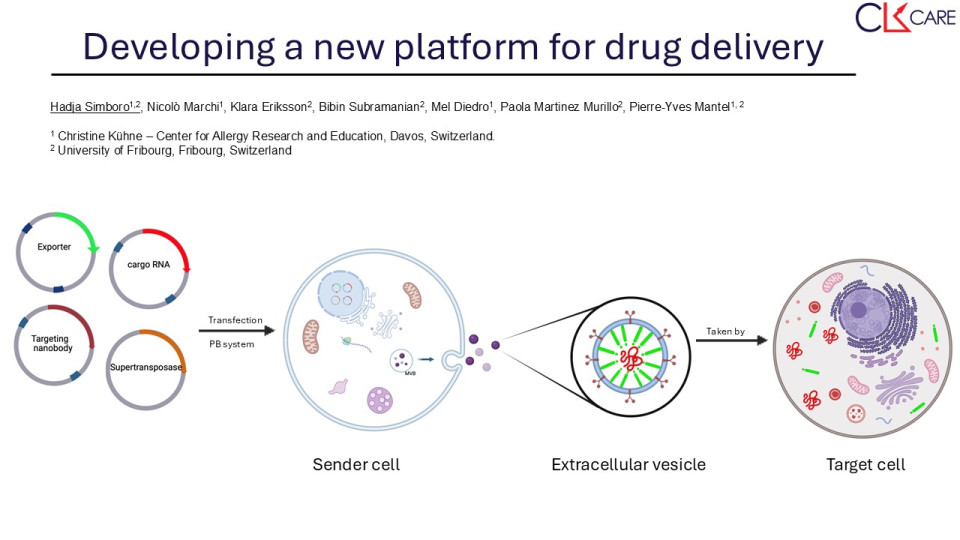
The landscape of drug delivery has evolved significantly with the development of various synthetic nanoparticulate systems aimed at enhancing the properties of therapeutics. While liposomes, polymeric nanoparticles, and dendrimers offer targeted drug release, they come with limitations. Viral vectors, including adeno-associated viruses (AAVs), adenoviruses (Ads), and lentiviruses (LVs), have shown efficiency in gene delivery but face safety concerns and production challenges. Lipid nanoparticles (LNPs), such as liposomes and solid lipid nanoparticles (SLNs), offer advantages like biocompatibility and enhanced drug-carrying capacity but struggle with precise control over release kinetics and limited loading capacity. Extracellular vesicles (EVs) are a heterogeneous group of small vesicles released by almost every cell that can transfer functional cargoes between cells. Comprising exosomes, microvesicles, and apoptotic bodies, EVs circulate in the body and cross different barriers to reach specific cells in tissues. They reach target cells through fusion with the cellular membrane of recipient cells, endocytosis, and also receptor-ligand interaction ways. EVs contain many different molecular cargoes such as proteins and nucleic acids derived from their cells of origin. Therefore, EVs can be seen as a natural drug delivery system. Of particular interest, are EVs released by red blood cells (RBCs). Indeed, RBCs harbor several advantages since they are poorly immunogenic and do not contain DNA.
Here, we propose to develop a drug delivery system based on EVs. We are focusing on the optimization of cargo loading and targeting by using HEK and K562 cells. Ultimately, we want to use EVs secreted by red blood cells derived from induced pluripotent stem cells.
For the stable expression of the exporter, we are generating plasmids that contain the exporter gene and express GFP reporter genes, that will be used for the selection of transfected cells. Then we will use the Cre recombinase as a cargo. HEK293 and K562 cells will be transduced and the cells expressing the transgenes will be sorted by Flow Cytometry. We will also use RNA sequencing to measure the specific enrichment of the RNA cargo in EVs versus the total RNA content of the cells. Then we will generate cell lines expressing the targeting nanobodies: anti-EGFR and anti-Her2 nanobodies. EV binding to cells expressing the EGFR or HER2 will be tested by flow cytometry and microscopy. To test the delivery of Cre-encoding mRNA by the donor cells, we will measure the export of RNA from the donor cells and the transfer to the receiver cells, which will activate GFP expression upon Cre recombination.