Immunomodulation is becoming more widely recognized as an important element in bone healing, sparking growing interest in osteoimmunology. This new field explores the complex relationship between the immune system and how bones develop and maintain their strength. Key to keeping bones dense, strong, and in good health is the careful balance between the processes that break down bone (bone resorption) and those that build it up (bone formation). Understanding this balance and its modulation by immune processes is crucial for advancing treatments that enhance skeletal health and recovery from bone-related injuries.
This project is focused on developing innovative bone-forming substitutes and using high-throughput techniques to investigate their immunomodulatory effects on immune cells. Peripheral blood mononuclear cells (PBMC) cultures were meticulously optimized using a variety of commercially available compounds, such as beta-tricalcium phosphate, tyramine-modified hyaluronic acid (THA) gel, both filtrated and unfiltered bone particles (in monolayer and transwell cultures), agarose, fibrin sealant (Evicel), and their respective combinations. Phytohemagglutinin and a mixture of anti-CD2, anti-CD3, and anti-CD28 monoclonal antibodies were used as positive controls for stimulation in the assay. The experimental procedures included assessing proliferation through the integration of tritiated thymidine and microscopic observations, evaluating cell viability using propidium iodide in flow cytometry, and conducting targeted proteomics with the Proximity Extension Assay.
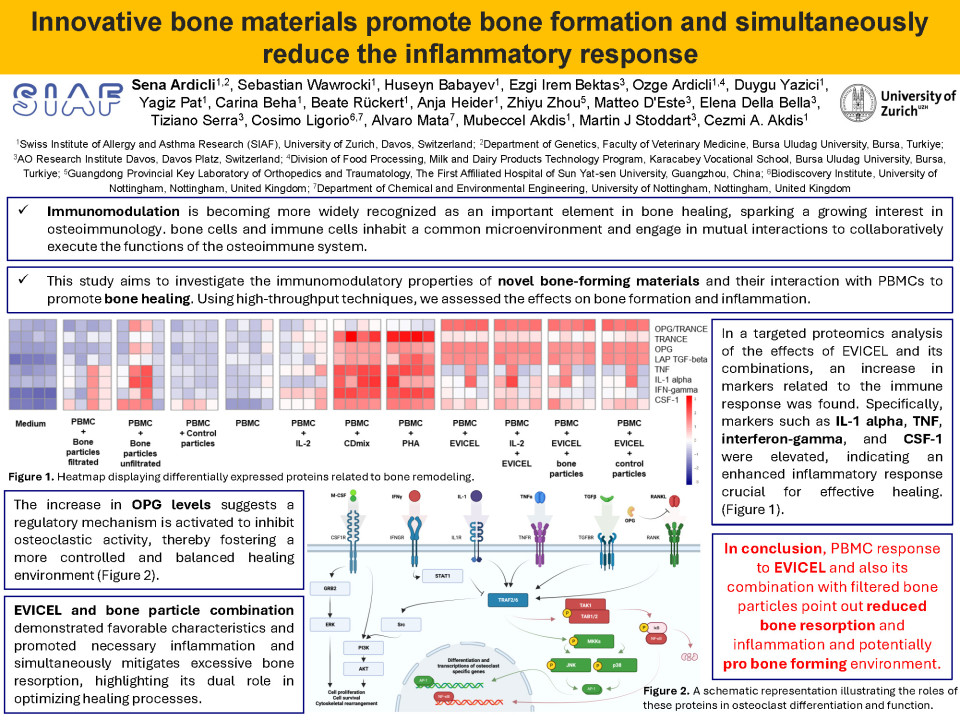
The results indicated a modest enhancement in cell proliferation when subjected to specific test materials, including THA gels, filtrated bone particles, and fibrin sealant. Conditions involving agarose resulted in a relatively increased level of proliferation. In the targeted proteomics analysis (OLINK T-92, inflammation and immune response panels), combinations of fibrin sealant and bone particles resulted in the upregulation of proteins linked to bone remodeling and reduced inflammatory response. It is worth noting that conditions involving agarose and bone particles did not produce significant changes in our biomarker panels. Regarding the ultimate results from proteomics analysis, we developed specialized panels (categorized as the bone remodeling and immune response panels) and examined the profiles displayed under various conditions in relation to these markers. We identified pathways related to the matrisome, plasmacytoma, apoptosis, and immune responses for fibrin sealant, both alone and in combination with filtrated bone particles. For optimal bone healing, a balanced dynamic between osteoblastogenesis and osteoclastogenesis is essential. Understanding the molecular basis of bone formation offers insights into the assessment of bone-related diseases and provides potential therapeutic targets for interventions aimed at regulating bone remodeling. In our study, the combination of fibrin sealant with bone particles exhibited beneficial properties in the analysis of both bone remodeling and immune response panels, suggesting its potential to enhance bone formation and modulate immune activities. In this context, fibrin sealant-related conditions exhibited desired bone remodelling dynamics, regarding CSF-1, IFN-gamma, IL-1 alpha, LAP TGF-beta-1, OPG, TNF, and TRANCE expression. This was also corroborated by downregulation in the NT-3, TSLP, LIF, TGFα, STC1, TNFSF14, UPA, OSM, β-NGF, and FGF21 while upregulation in the TRAIL, CDCP1, MMP1, MMP10, MCP1, VEGFA, FGF19, LIF-R, SCF, CST5, and FGF23 in the fibrin sealant and bone particles combination.
In conclusion, the response of PBMCs to fibrin sealant and its combination with filtered bone particles indicates a decrease in bone resorption and inflammation, suggesting a potentially favorable material for bone formation.
Quiz question
What is the primary PBMC response to EVICEL, as well as to its combination with filtered bone particles?
It leads to increased bone resorption and inflammation, indicating that it is an unsuitable material for bone healing.
This combination provides reduced bone resorption and inflammation and a potentially pro-bone-forming environment.
This combination appears to be inert and has no significant effect on PBMCs.